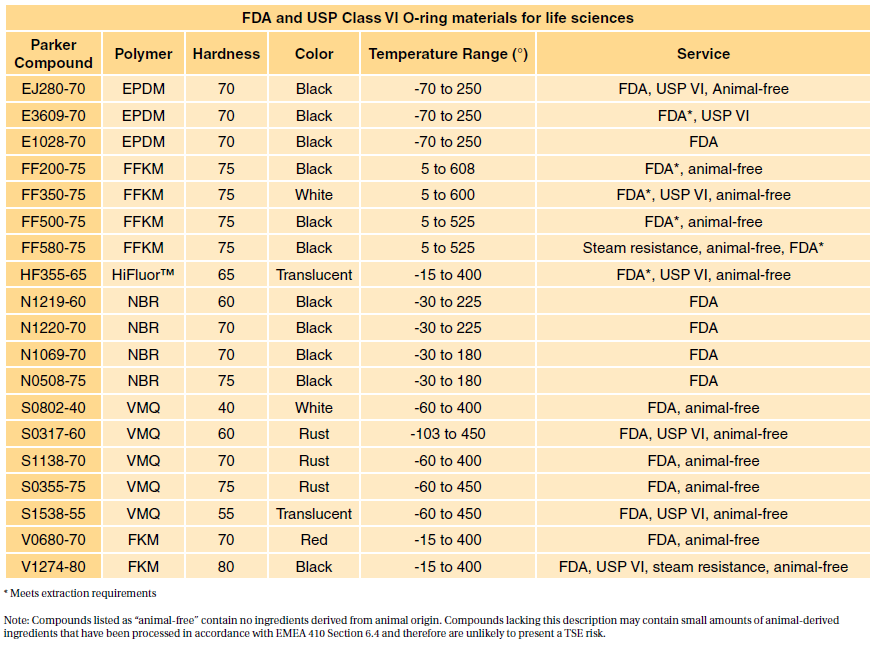
usp class vi vs iso 10993
However Class VI also requires subacute toxicity and implantation. So does ISO 10993.
General Chapters 1031 The Biocompatibility Of Materials Used In Drug Containers Medical Devices And Implants
ISO 10993 is designed for medical products that remain permanently or for a very long time in the human body so for.

. USP Class VI ISO 10993-5 Cytotoxicity In-Vitro ISO 10993-3 Ames Genotoxicity ISO 10993-11 Systemic Toxicity In-Vivo ISO 10993-4 Hemolysis Indirect European Pharmacopeia 329. USP Class VI demands an intracutaneous irritation test. USP Class VI testing is conducted by producing an extract of the product with different extraction fluids such as.
In fact USP Class VI is sometimes seen as a minimum. USP Class VI demands an intracutaneous irritation test. USP Class VI refers to one of the six designations for plastics from General Chapter of the United States.
Typically the terms USP Class VI or ISO 10993 materials are used. Below youll find a list of all posts that have been tagged as USP Class VI ISO 10993 vs. Take an ASTM D2000 call out.
Sumeet mixie jars in hyderabad. You might establish biocompatibility via making the device of a Recognized Consensus. USP class qualification was a key method for establishing material biocompatibility at least as far back as 1976 until the.
May 1 2009. The most stringent Class VI requires three types of tests. Biocompatibility - USP Class VI vs.
USP Class VI vs. We carry a wide range of materials from the worlds top medical polymers suppliers including USP Class VI and ISO 10993 certified biocompatible resins with full FDA. USP class VI versus ISO 10993.
Both ISO 10993 and USP Class VI define testing requirements for biocompatibility the ability of a material to perform a desired function without causing adverse effects on the. Medical Molding and Biocompatible Rubber. Rob Pruyn August 5 2020 Custom Products.
ISO 10993 is a 20-part standard that. Iso 10993 vs. Medical Molding and Biocompatible Rubber.
Though not a limited series of tests some biocompatibility requirements for medical devices may exceed the testing performed in USP Class VI. Statistical software in research methodology. In an effort to standardize biocompatibility testing worldwide the International Standards Organization ISO developed ISO 10993.
Tyre prices south africa. These international standards refer to the testing requirements for bio-compatibility most commonly used in the medical sector and meet very high standards of. Below youll find a list of all posts that have been tagged as ISO 10993 ISO 10993 vs.
Usp class vi and iso 10993. Testing for proving food safety on USP class. ISO 134852016 - Medical Device Quality Management Systems.
A more rigorous standard for the biological. USP Class VI and ISO 10993. If yes to the first question then USP Class VI is not a relevant qualification for it.
Unlike other rubber standards theres no one standard that engineers use for an approval. Skf 6000 2z c3. Rob Pruyn August 5 2020 Custom.

What Is Iso 10993 How Is It Different From Usp Class Vi Ppt Download
Biocompatibility Of Plastics Zeus
A Biocompatible Polycarbonate 3d Printing Material Stratasys

Medical Grade Cyanoacrylate Super Glue Iso 10993 And Usp Class Vi
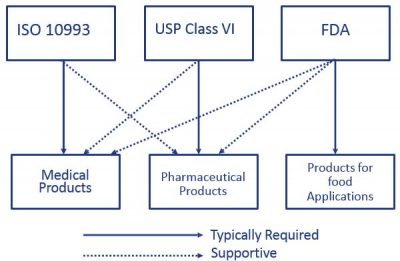
Material Selection Medical Injection Molding Xcentric Mold

Usp Class Vi Certification Presco Marking Products And Engineered Films
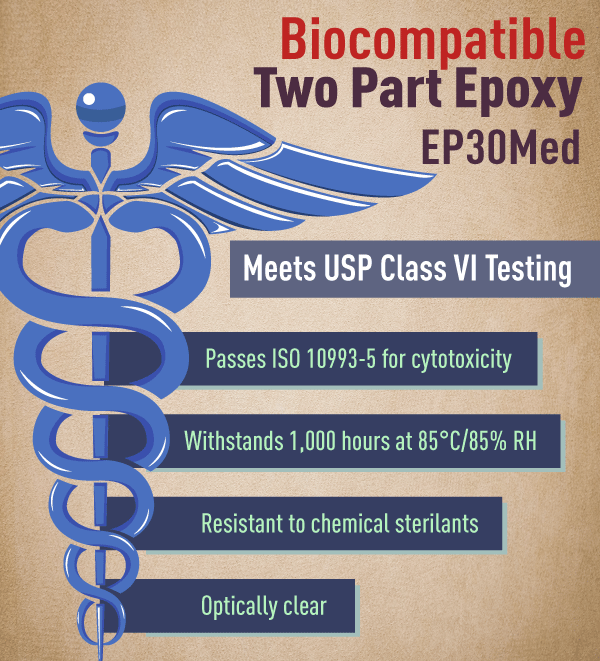
Ep30med Low Viscosity Epoxy Meets Usp Class Vi Specifications Quote Rfq Price And Buy

Regulatory Guidelines For Biocompatibility Safety Testing Mddionline Com
Master Bond Introduces A New Addition Cured Silicone That Meets Usp Class Vi And Iso 10993 5 Specifications For Biocompatibility And Cytoxicity
Biocompatibility Planning Tool Biopt Pacific Biolabs

What Is Iso 10993 How Is It Different From Usp Class Vi Ppt Download

Looking Beyond Usp Class Vi Testing What Is Usp 87 Testing Holland Applied Technologies

Polyone Expands Lineup Of Pre Certified Biocompatible Healthcare Colorants And Additives Avient

Usp Class Vi Adi Free What Does It All Mean
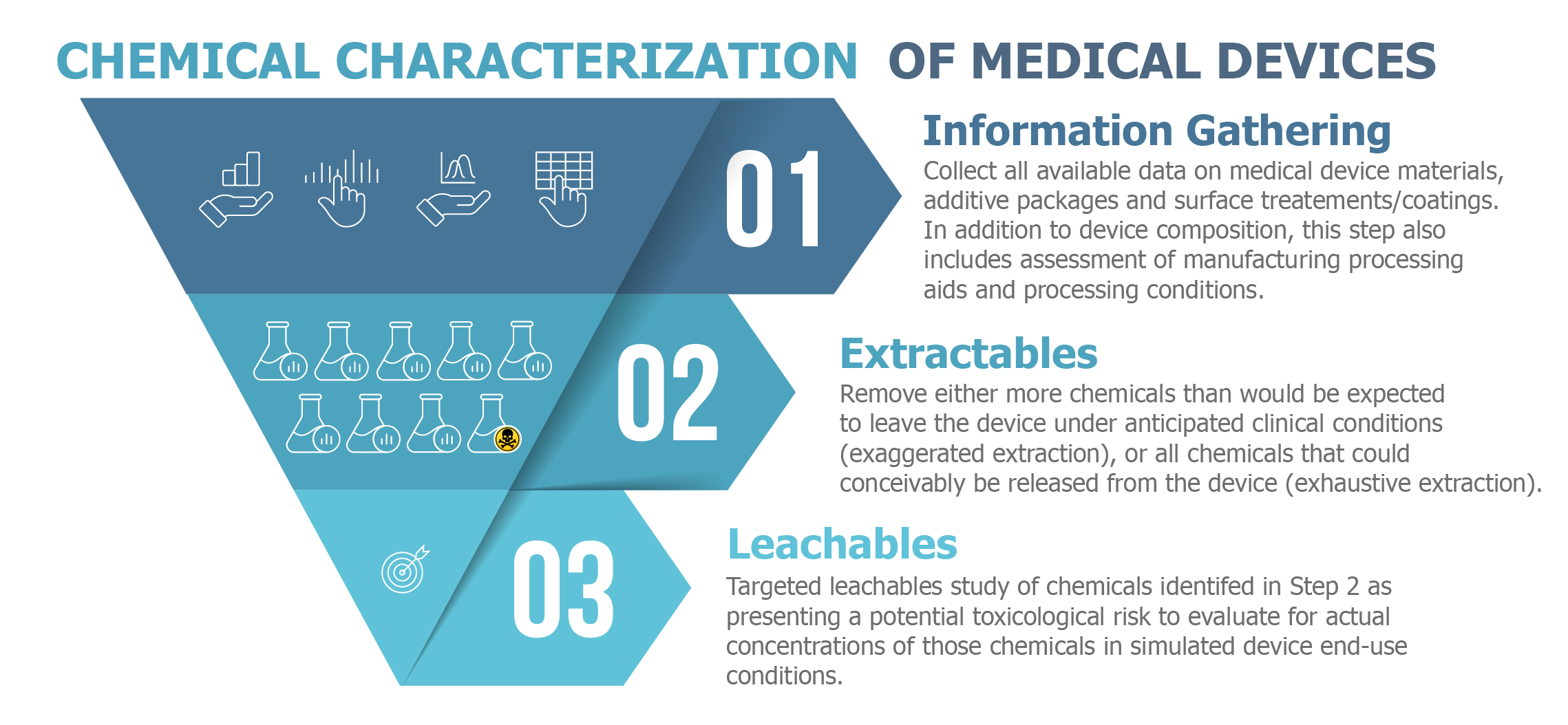
Seismic Shifts In Iso 10993 18 2020

Why Biocompatibility Should Be Addressed By Every Medical Device Company
Medical Device Biocompatibility Cdg Whitepapers

Pc Iso Polycarbonate Iso 10993 Usp Class Vi 3d Printing Material Xometry Europe